
Representation of the nickel coordination environment. The distances... | Download Scientific Diagram
2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0277538713008310-fx1.jpg)
Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect
![Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution. Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution.](https://haygot.s3.amazonaws.com/questions/2015518_249889_ans_bbd2828107e04c46b63fdcb0f0c04801.jpg)
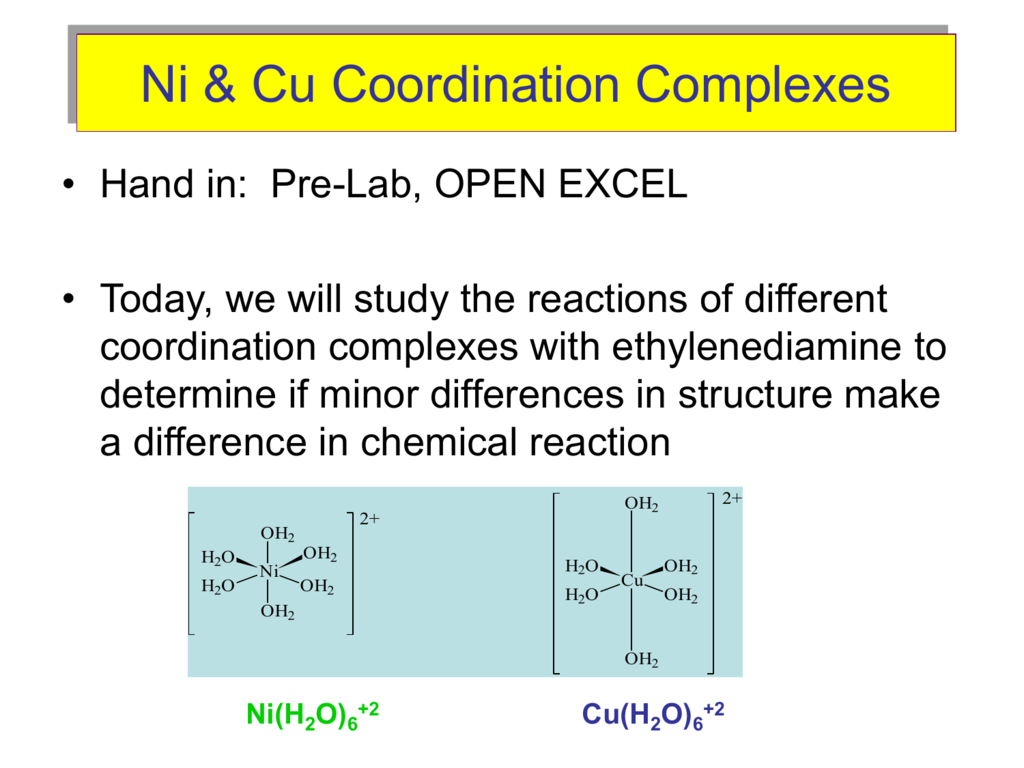
Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution.
![IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2 IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2](https://journals.iucr.org/e/issues/2020/03/00/xi2016/xi2016scheme1.gif)
IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2
Crystal structure of Ni-EDPA: (a) View of the cation-anion pair; (b)... | Download Scientific Diagram
![Figure 2 from Optical spectra of the complexes [ M ( H 2 O ) 6 ] 2 + and MSO 3 – ( H 2 O ) 2 ( M = Ni 2 + ) | Semantic Scholar Figure 2 from Optical spectra of the complexes [ M ( H 2 O ) 6 ] 2 + and MSO 3 – ( H 2 O ) 2 ( M = Ni 2 + ) | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/aafdd9c3574f95c99a547bef31f5bac81718fa83/2-Figure2-1.png)
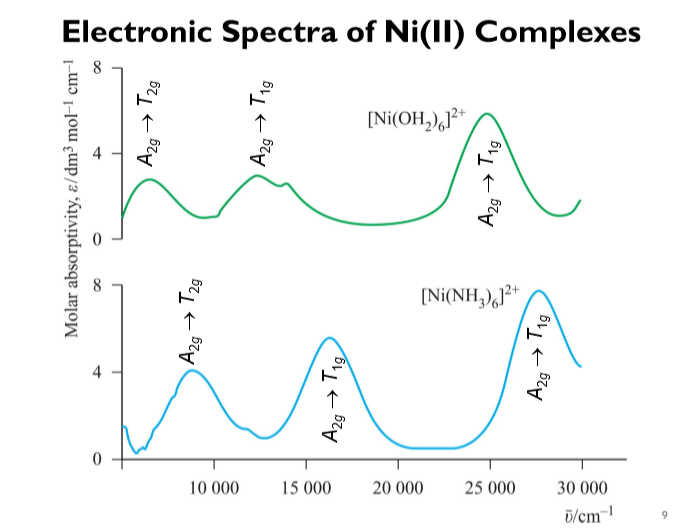
Figure 2 from Optical spectra of the complexes [ M ( H 2 O ) 6 ] 2 + and MSO 3 – ( H 2 O ) 2 ( M = Ni 2 + ) | Semantic Scholar
2. All atoms, except the | Download Scientific Diagram Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram](https://www.researchgate.net/profile/Duncan-Gregory/publication/269400063/figure/fig5/AS:667921089052683@1536256205687/Representation-of-the-unit-cell-of-NiH2O6NO32-All-atoms-except-the_Q640.jpg)
Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram
![A question from my textbook: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless. Explain. : r/chemhelp A question from my textbook: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless. Explain. : r/chemhelp](https://external-preview.redd.it/VDYxIhyF0tX1EvHO09Izi030EWZe0sji-zLW3q5xn38.jpg?auto=webp&s=05f1b2c012b7b91e46340d66d18806bcaa6e97b2)
A question from my textbook: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless. Explain. : r/chemhelp
![Why a solution of [Ni(H2O)6]^2 + is green while a solution of [Ni(CN)4]^2 - is colourless? (At. no. of Ni = 28) Why a solution of [Ni(H2O)6]^2 + is green while a solution of [Ni(CN)4]^2 - is colourless? (At. no. of Ni = 28)](https://d1hj4to4g9ba46.cloudfront.net/questions/1194031_1237569_ans_624888e40c0e480caf24f69d44c6e352.jpg)
Why a solution of [Ni(H2O)6]^2 + is green while a solution of [Ni(CN)4]^2 - is colourless? (At. no. of Ni = 28)
What is the hybridization, transition, spin, colour, magnetism, and geometry of [Ni(H2O) 4] +2? - Quora
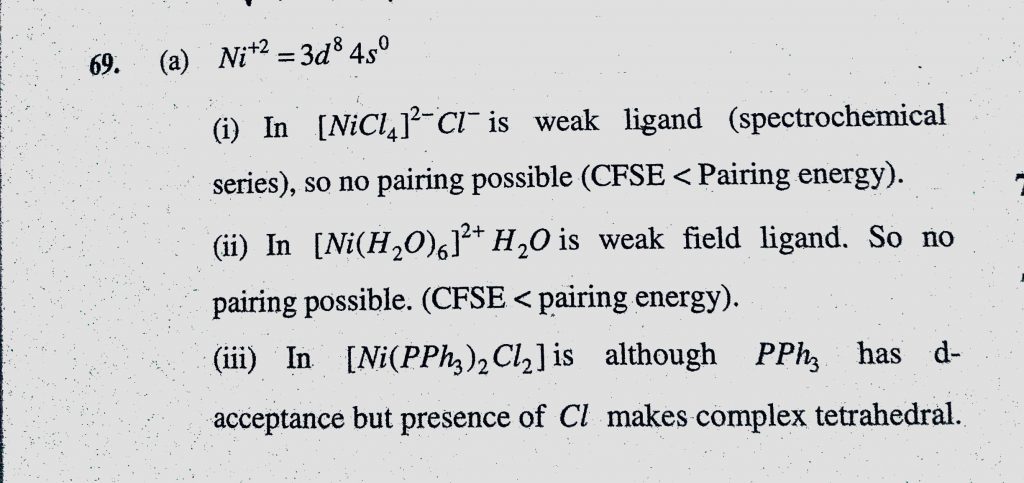


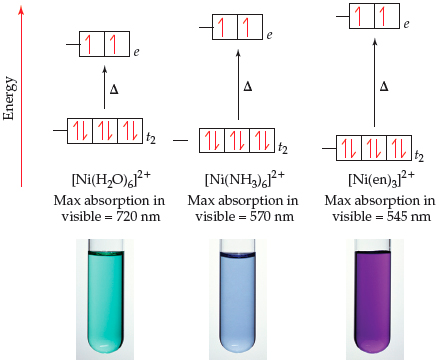
![What is the hybridization of Ni and structure of [Ni(H2O) 6] 2+? - Quora What is the hybridization of Ni and structure of [Ni(H2O) 6] 2+? - Quora](https://qph.cf2.quoracdn.net/main-qimg-fa42f87b83e5b7668101dfb77e274e3c.webp)
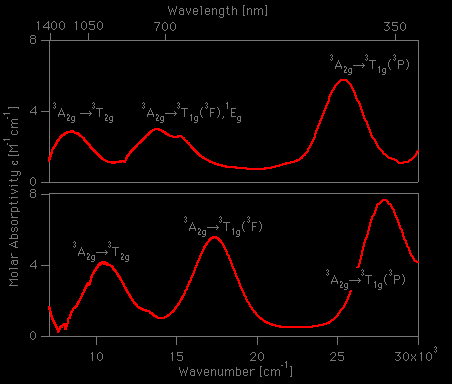

![Solved 20. When H2O in the [Ni(H2O)6]2+ complex ion is | Chegg.com Solved 20. When H2O in the [Ni(H2O)6]2+ complex ion is | Chegg.com](https://media.cheggcdn.com/media%2F782%2F7824ed9d-14a5-4257-bc8c-05dc5a9cf139%2FphpvCnudZ.png)


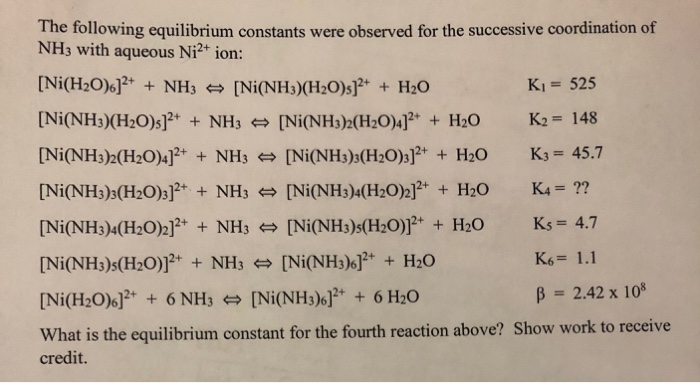

2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar FIR Spectra of [Ni(H2O)6](ClO4)2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ecaf6b0fee69dc0a5060cdb8d7e5d0c7d395a06e/4-Table1-1.png)

![A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain. A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain.](https://haygot.s3.amazonaws.com/questions/1955044_1567628_ans_f710a7dfc21d4ea7a5d920b41112c727.jpg)